Courses
- CH 621 Electrochemistry (Graduate Course)
- CH 223 Quantitative Analysis
- CH 102 General Chemistry
- CH 101 General Chemistry
CH 621 Trends In Analytical Chem (Electrochemistry)
Prerequisites
Prerequisites for this course are general chemistry (CH101, 102). All students are assumed to have some background in half reaction potentials, standard potentials, redox reactions, and electroanalytical chemistry at the general level of undergraduate courses (e.g., quantitative analysis). Some ability in computer programming, at the minimum use of spreadsheets, like Excel (to carry out the digital simulations taught in the course) is also assumed.
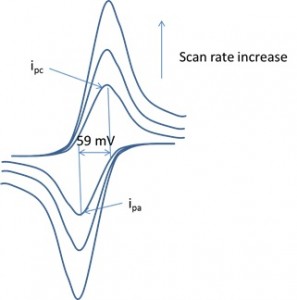
Course Description
This course is designed to teach the fundamental aspects of electrochemistry and the application of electrochemical methods to chemical/engineering problems. Special emphasis will be given to the study of electrode reaction mechanisms and the interpretation of electrochemical results (e.g., cyclic voltammetry) for organic and inorganic systems. A rigorous consideration of voltammetric and coulometric methods and several topics of interest in electrochemistry including modified electrodes, photoelectrochemistry, scanning electrochemical microscopy and electrogenerated chemiluminescence etc will be undertaken. We will also introduce the concept of AC impedance, solar cell and fuel cell at the end of the course. There are hands-on activities (e.g., CVs, solar cell, electrolysis, fuel cell, energy transformation, rotating disc electrode) provided to enhance the learning.
Student Learning Outcomes
This course will help graduate students interpret various electrochemical results (e.g., cyclic voltammetry) for organic and inorganic systems. Students are expected to be capable of using digital simulation and electrochemistry theory to understand electrochemical phenomenon; secondly, students will be able to apply electrochemical methods to solve chemistry and chemical engineering problems existing at a solid-liquid interface; third, student will have a comprehensive understanding of analytical chemistry application of electrochemistry and learn how electrochemistry is used in sensors for ultrasensitive detection; Several types of devices such as fuel cell, light emitting electrochemical cell and solar cell will be introduced in order to enhance their understanding of the principles of electrochemical systems. Students are expected to have the ability to develop their own interests further based on what we will learn through the course by reading literature and conducting research in these important fields.
Required Texts
UA Supply Store Textbook Information
J. Bard and L. R. Faulkner, Electrochemical Methods, Second edition, John Wiley & Sons, Inc. Two copies of this book are available in Rodgers Library. The second and/or third year chemistry graduate students may have their own copies.
Blackboard
The UA blackboard site (https://ualearn.blackboard.com/webapps/login/) contains a variety of useful course materials, including teaching slides, reading materials and software for digital simulations.
References
The following references are available at Rodgers Library. These are recently purchased materials for your study and research by the course instructor.
Encyclopedia of Electrochemistry, Volume 1-10
Thermodynamics and Electrified Interfaces; Interfacial Kinetics and Mass Transport; Instrumentation and Electroanalytical Chemistry; Corrosion and Oxide Films; Electrochemical Engineering; Semiconductor Electrodes and Photoelectrochemistry; Inorganic Electrochemistry; Organic Electrochemistry; Bioelectrochemistry; Modified Electrodes.
Outline of Topics
| Topic |
|---|
| Introduction, Standard electrode potential, cell thermodynamics effect of concentration on electrode potential (The Nernst equation) |
| Lab 1 Voltage measurement of galvanic elements Potential/current control Electrochemical Methods I Lab 2 Electrode Potential Measurement in 3-electrode configuration (ref, counter and working) |
| Double layer structure, potential/current control Electrochemical Methods |
| Mass transfer |
| Kinetic of electrode reactions |
| Potential/current control Electrochemical Methods II Lab 3 Cyclic Voltammetry |
| Theoretical: Laplace Transform for control potential methods |
| Digital simulation |
| Linear Sweep and Cyclic Voltammetry Lab 4 Rotating Disc Electrode |
| Digital simulation of Linear Sweep and Cyclic Voltammetry |
| Double Layer Structure and Adsorption – Modified Electrodes |
| Analysis of Electrode Reaction Mechanisms |
| Nanoelectrochemistry and trace analysis using electrochemical methods |
| Modified electrode for electrochemical sensing |
| Spectroelectrochemistry |
| Introduction to electrogenerated chemiluminescence (ECL) and OLEDs |
| Current status of ECL and applications Lab 5. chemiluminescence and electroluminescence |
| Microelectrode, Scanning electrochemical microscopy (SECM) using microelectrode, Application of SECM (Lab tour for SECM) |
| Photoelectrochemistry Lab 6 Alternative Renewable Energy: Solar Water Splitting for Hydrogen |
| Direct water splitting at nanoelectrodes using solar energy |
| Dye sensitized electrochemical solar cell |
| Supercapacitors |
| AC Impedance |
| AC Impedance in solar cell, batteries and fuel cell Lab. 7 Al-air battery |
| Other E-chem methods (CC, CA, Stripping, LSV and other pulse techniques) |
| Student presentations and papers |
